
Molisch’s Test: Objectives, Principle, Reagents, Procedure and Result
Objective:
- to identify carbohydrate from other biomolecules
Principle of Molisch’s test:
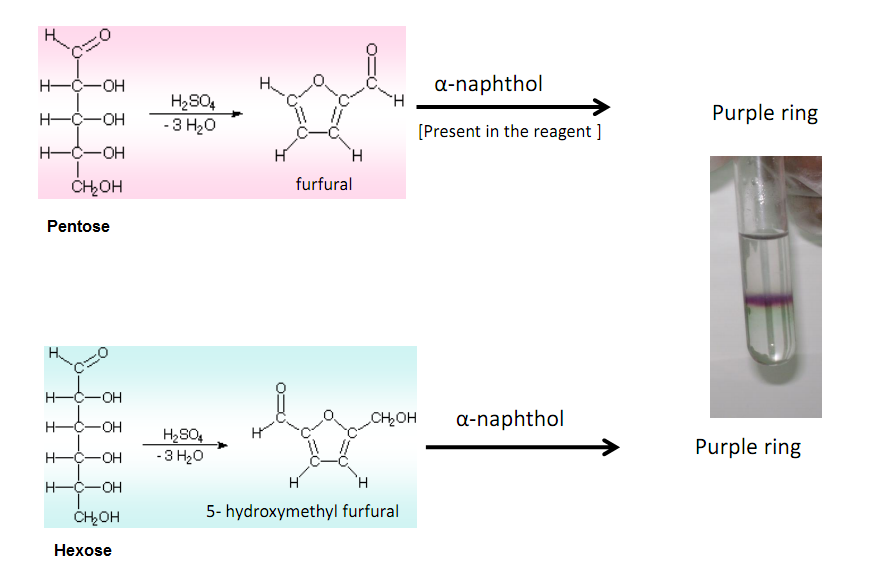
Molisch’s test is a general test for all carbohydrates. In this test, carbohydrates when reacted with conc. H2SO4 get dehydrated to form furfural and its derivatives.
When monosaccharide are treated with conc H2SO4 or conc HCl, -OH group of sugar are removed in the form of water and furfural is formed from pentose sugar and hydroxymethyl furfural is formed from hexose sugar. These products reacts with sulphonated α- naphthol to give a purple (violet red) colored complex.
Reagents for Molisch’s test:
- test solution: 5 % Glucose, 5 % Sucrose, 5 % Starch
- Molisch’s reagent (5 % α naphthol in ethanol)
- H2SO4
- Dry test tubes
- pipettes
Procedure of Molisch’s test:
- Take 2ml of sample in dry test tube.
- Take 2ml of distilled water in another tube as control.
- Add 2-3 drops of Molisch’s reagent to the solution.
- Gently pipette 1ml conc. H2SO4 along the side of the tube so that two distinct layers are formed.
- Observe color change at the junction of two layers.
- Appearance of purple color indicates the presence of carbohydrates.
Result Interpretation of Molisch’s test:
Positive Molisch’s test: purple color complex
Negative Molisch’s test: no purple color

