
Ames test
Principle
- Ames test is developed by Bruce N. Ames in 1970s to test for determining if the chemical is mutagens. This test is based on the principle of reverse mutation or back mutation. So, the test is also known as bacterial reverse mutation assay.
- Test organism: Ames test uses several strains of bacteria (Salmonella, E.coli) that carry mutation. Eg A particular strain of Salmonella Typhimurium carry mutation in gene that encodes histidine. So it is an auxotrophic mutant which loss the ability to synthesize histidine (an amino acid) utilizing the ingredients of culture media. Those strains are known as His- and require histidine in growth media.
- Culturing His- salmonella is in a media containing certain chemicals, causes mutation in histidine encoding gene, such that they regain the ability to synthesize histidine (His+) This is the reverse mutation. Such chemicals responsible to revert the mutation is actually a mutagen. So, this Ames test is used to test mutagenic ability of varieties of chemicals.
Procedure: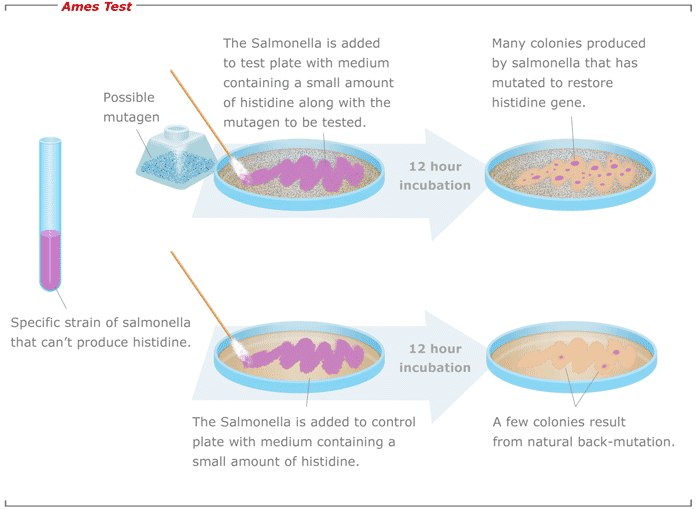
Figure: Ames test
i ) Isolate an auxotrophic strain of Salmonella Typhimurium for histidine. (ie. His-ve)
ii) Prepare a test suspension of his-ve Salmonella Typhimurium in a plain buffer with test chemical (let’s say 2-aminofluorene). Also add small amount of histidine.
Ps: small amount of histidine is required for initial growth of bacteria. Once histidine is depleted only those bacteria mutated to gain the ability to synthesize histidine form colony.
iii) Also prepare a control suspension of His-ve Salmonella Typhimurium but without test chemicals.
iv) Incubate the suspensions at 37°C for 20 minutes
v) Prepare the two agar plate and spread the suspension on agar plate.
vi) Incubate the plates at 37°C for 48 hours.
vii) After48 hours count the number of colonies in each plate. The mutagenicity of chemicals is proportional to number of colonies observed. If large number of colonies on test plate is observed in comparison to control, then such chemical are said to be mutagens.
*Very few number of colonies can be seen on control plate also. This may be due to spontaneous point mutation on hisidine encoding gene.
Application:
- The practical application of Ames test is to screen chemical mutagens that causes mutation and are carcinogenic to human and animals. Some of the chemicals used as food additive (AF-2), flavoring agent (Safrole) are mutagenic as well carcinogenic.
- Isoniazid; an anti TB drug is also mutagens.
- Ames test adopted to use eukaryotic cell culture, yeast cell, as well as animal model to test mutagens. Since, Salmonella is not a best test organism to test mutagens for Human. Certain chemicals initially are not mutagens to human but convert into mutagens when metabolized (acted upon by body enzymes). For example; sodium nitrate (NaNO3) is not mutagens until it is acted upon by HCL in stomach to form Nitrous oxide HNO2 (a potent mutagen).
- Ames test can detects Suitable mutants in large population of bacteria with high sensitivity.
- It is test for mutagenicity not carcinogenicity. However, most of the mutagens (more than 90%) detected by Ames test are responsible to cause cancer.
- It is a bacterial reverse mutation assay. So the defective gene of bacteria can be mutated into functional gene.
References
- http://www.encyclopedia.com/environment/encyclopedias-almanacs-transcripts-and-maps/ames-test
- http://www.biology-pages.info/A/AmesTest.html
- https://www.mun.ca/biology/scarr/4241_Ames_Test.html
- https://online.science.psu.edu/micrb106_wd/node/6162
- https://ntp.niehs.nih.gov/testing/types/genetic/invitro/sa/index.html
- http://www.genetics-gsa.org/education/pdf/GSA_DeStasio_Ames_Student_Resources.pdf
- http://www.cyprotex.com/toxicology/genotoxicity/amestest
- https://www.awqc.com.au/our-services/product-testing/the-ames-test
- http://www.eurofins.com/biopharma-services/discovery/services/adme-tox/in-vitro-toxicity/genotoxicity/ames-test/
