Antigen processing and Antigen presentation
- Antigen processing is a metabolic process that digests the proteins into peptides which can be displayed on the cell membrane together with a class-I or class-II MHC molecules and recognized by T-cells.
- Antigen presentation is the process by which certain cell in the body especially antigen presenting cells (APCs) express processed antigen on their cell surface along with MHC molecules in the form recognizable to T cell.
- If antigen is presented along with class-I MHC molecule, it is recognized by CD8+ Tc-cell and if presented along with class-II MHC molecule, it is recognized by CD4+ TH cells.
On the basis of types of antigen to be processed and presented, antigen processing and presenting pathway are of two types:
Cytosolic pathway of antigen processing and presentation
- Cytosolic pathway processed and presented the endogenous antigens i.e. those generated within cell eg. Viral infected cells, tumor cells and intracellular pathogens (M. tuberculosis, Histoplasma capsulatum).
- The processed antigen is presented on the cell membrane with MHC-class I molecule which is recognized by CD8+ Tc-cell for degradation.
Steps involved in cytosolic pathways are:
- Proteolytic degradation of Ag (protein) into peptides
- Transportation of peptides from cytosol to RER
- Assembly of peptides with class I MHC molecules
i. Proteolytic degradation of proteins into peptides:
- Intracellular proteineous antigen are larger in size to be bound to MHC molecule.
- So, it is degraded into short peptides of about 8-10 amino acids.
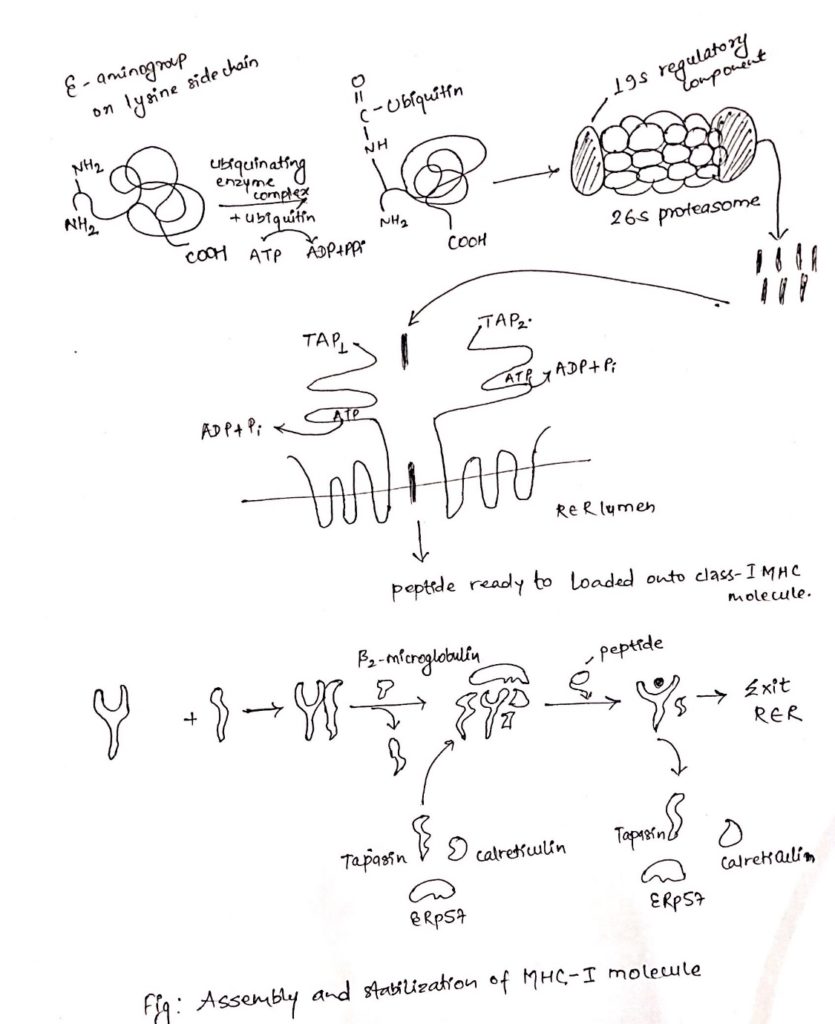
- These proteins are degraded by cytosolic proteolytic system present in cell called proteasome.
- The large (20S) proteasome is composed of 14 sub-units arranged in barrel-like structure of symmetrical rings.
- Some, but not all the sub-units have protease activity.
- Proteins enter the proteasome through narrow channel at each end.
- Many proteins targeted for proteolysis have a small protein called ubiquitin attached to them.
- Ubiquitin attached to them ubiquitin-protein complex consisting of 20S proteasome and 19S regulatory component added to it.
- The resulting 26S proteasome cleaves peptide bonds which is ATP-dependent process.
- Degradation of ubiquitin protein complex is thought to occur within the central hollow of the proteasome to release peptides.
ii. Transportation of peptides from cytosol to Rough Endoplasmic Reticulum (RER):
- Peptides generated in cytosol by proteasome are transported by TAP (transporter associated with antigen processing) into RER (Rough endoplasmic reticulum) by a process which require hydrolysis of ATP.
- TAP is membrane spanning heterodimer consisting of two proteins, TAP1 and TAP2.
- TAP has affinity for peptides having 8-16 amino acids.
- The optimal peptide length required by class-I MHC for binding is nine, which is achieved by trimming the peptides with the help of amino-peptidase present in RER. Eg. ERAP.
- In addition to it, TAP favor peptides with hydrophobic or basic carboxyl terminal amino acids, that preferred anchor residues for class-I MHC molecules.
- TAP deficiency can lead to a disease syndrome that has both immune-deficiency and auto-immunity aspects.
iii. Assembly of peptides with class-I MHC molecule:
- Like other proteins, the α-chain and β2 microglobulin components of the class-I MHC molecule are synthesized on polysome along the rough endoplasmic reticulum.
- Assembly of these components into stable class-I MHC molecule that can exit the RRE require binding of peptides into peptide binding groove of class-I MHC molecules.
- The assembly process involves several steps and needs help of molecular chaperone.
- The first molecular chaperone involved in assembly of class-I MHC is calnexin.
- It is a resident membrane protein of RER.
- Calnexin associated with free class-I α-chain and promotes its folding.
- When β2-microglobulin binds class-I α-chain, calnexin is released and class-I MHC associates with another chaperone calreticulin and tapasin (TAP-associated protein).
- Tapasin brings TAP transporter carrying peptides to the proximity with class-I MHC molecule and allows to acquire the antigenic peptides.
- An additional protein with enzymatic activity, ERp57, form disulfide bond to tapasin and non-covalently associates with calreticulin to stabilize the interaction and allows release of MHC-I-class after acquiring antigenic peptides.
- As a consequence, the productive peptide binding with MHC of class-I releases from the complex of calreticulin, tapasin and ERp57, exit from RER and displays on the cell surface via golgi complex.
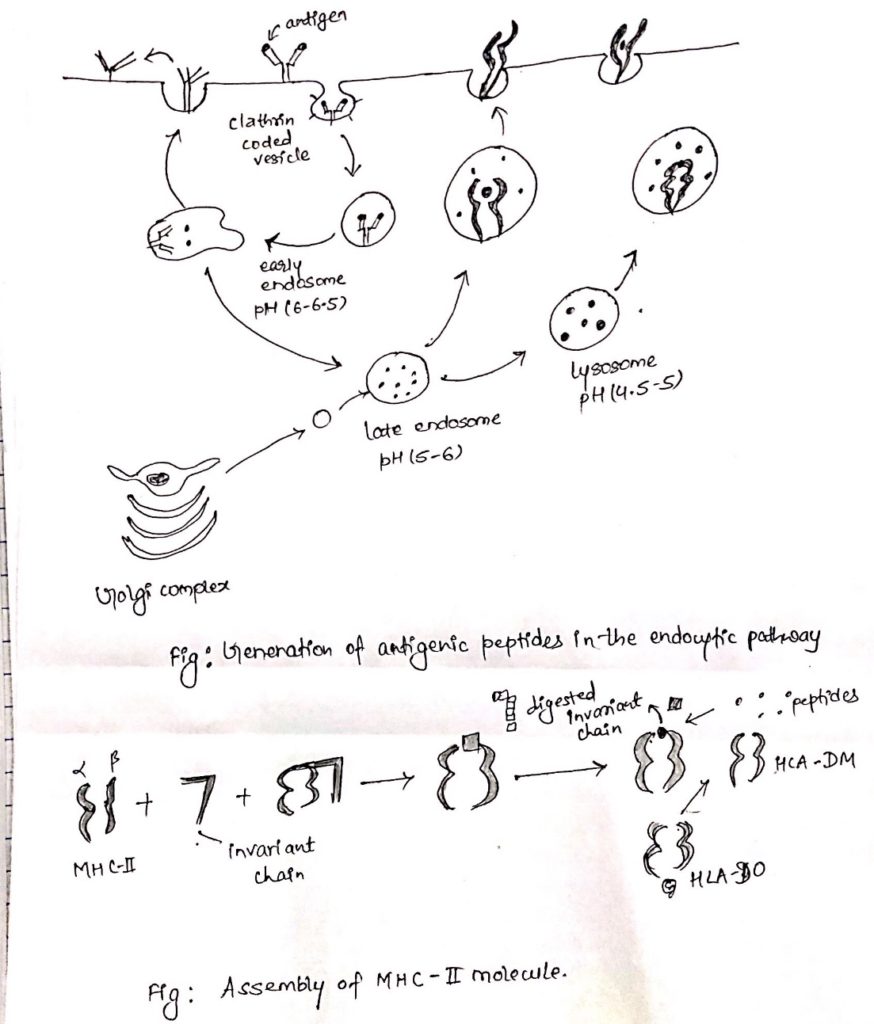
Endocytic pathway of antigen processing and presentation:
- The endocytic pathway processed and present the exogenous Ag. i.e. antigens generated outside the cells. E.g. Bacteria.
- At first APC phagocytosed, endocytosed or both, the antigen.
- Macrophage and dendritic cells internalize the antigen by both the process.
- While other APCs are non-phagocytic or poorly phagocytic. E.g. B cell internalize the antigen by receptor mediated endocytosis.
- Then antigen is processed and presented on the cell surface along with class-II MHC molecules which are recognized by CD4+ TH cell.
Steps involved in endocytic pathway:
- Peptide generation from internalized molecules (Ag) in endocytic vesicles.
- Transport of class-II MHC molecule to endocytic vesicles.
- Assembly of peptides with Class-II MHC molecules.
i. Peptide generation from internalized molecules (Ag) in endocytic vesicles:
- Once an antigen is internalized, it is degraded into peptides within compartments of endocytic processing pathway.
- The endocytic pathway appears to involve three increasingly acidic compartments, early endosomes (pH 6-6.5), late endosomes or endo-lysosome (pH 5-6) and lysosomes (pH 4.5-5).
- The internalized antigens move from early to late endosomes and finally to lysosomes, encountering hydrolytic enzymes and a lower pH in each compartment.
- Within the compartment, antigen is degraded into oligopeptides of about 13-18 residues.
- The mechanism by which internalized Ag moves from one endocytic compartment to next has not been clearly demonstrated.
- It has been suggested that early endosome move from periphery to inward to become late endosome and finally lysosomes.
- Alternatively, small transport vesicles may carry Ag from one compartment to next.
ii. Transport of class-II MHC molecule to endocytic vesicles:
- When class-II MHC molecules are synthesized within RER, three pairs of class-II αβ-chains associated with a pre-assembled trimer of a protein called invariant chain (Li, CD74).
- This trimeric protein prevents any endogenously antigen to bind to the cleft.
- The invariant chain consists of sorting signals in its cytoplasmic tail.
- It directs the transport of class-II MHC molecule to endocytic compartments from the trans-golgi network.
iii. Assembly of peptides with class-II MHC molecules:
- Class-II MHC-invariant chain complexes are transported from RER through golgi complex and golgi-network and through endocytic compartment, moving from early endosome to late endosome and finally to lysosome.
- The proteolytic activities increase in each compartment, so the invariant is slowly degraded.
- However, a short fragment of invariant chain remained termed as CLIP (Class-II associated invariant chain).
- CLIP physically occupies the peptide binding, cleft of class-II MHC molecule, presumably preventing any premature binding of antigenic peptides.
- A non-classical class-II MHC molecule known as HLA-DM is required to catalyze the exchange of CLIP with antigenic peptides.
- The reaction between HLA-DO, which binds to HLA-DM and lessens the efficiency of the exchange reactions.
- Conditions of higher acidity in endocytic compartment weakens the association of DM/DO and increase the possibility of antigenic peptide binding despite of DO.
- As with class-I MHC molecule, peptide binding is required to maintain the structure and stability of class-II MHC molecules.
- Once a peptide has bound the peptide-class II MHC complex is transported to the plasma membrane where neutral pH enables the complex to assume the compact and stable form.