
Chloride shift/Hamburger phenomenon
- The greater proportion (70%) of carbon dioxide is transported in the form of bicarbonates.
- The CO2 reacted with the water of the cytoplasm in the presence of enzyme carbonic anhydrase to form carbonic acid.
- The carbonic acid (H2CO3) is a weak acid, which undergoes partial dissociation to yield hydrogen ion (H+) and bicarbonate ion (HCO3-).
- The given reaction mostly occur inside RBCs, because the enzyme carbonic anhydrase is abundant there.
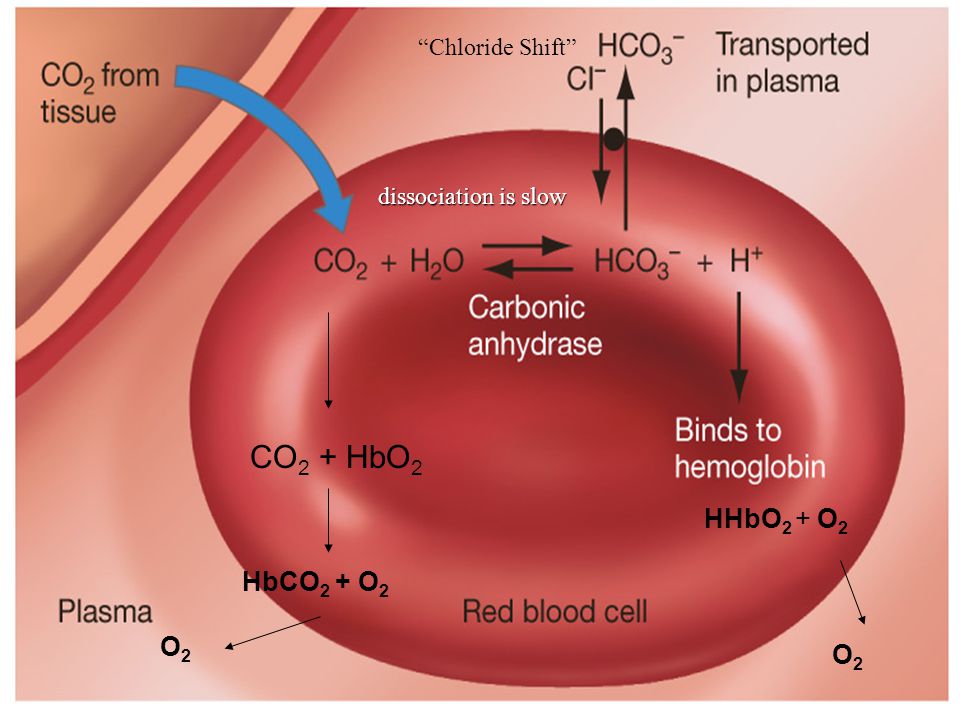
Fig. Diagrammatic representation of chloride shift.
- In RBCs, CO2 combines with water to from carbonic acid which dissociates to gives H+ ion and bicarbonate (HCO3-) ion in the presence of enzyme carbonic anhydrase.
- The bicarbonate ion then diffuses outside the RBC in the plasma and combines with Sodium ions to from Sodium bicarbonate (NaHCO3).
- Loss of bicarbonate ions from RBC causes positive charge inside RBC which is balanced by diffusion of chloride (Cl-) ion from plasma into the RBC.
- This exchange of Cl- ion and HCO3- ion between plasma and RBC is known as chloride shift.
- This phenomenon of chloride shift maintain the electrical neutrality of cell.
- This phenomenon is also known as Hamburger phenomenon.
- Reverse of chloride shift occurs in tissues.
