
What is enzyme inhibition?
- Enzyme inhibitors are the substance which when binds to the enzyme reversibly or irreversibly, decreases the activity of enzyme and the process is known as enzyme inhibition.
- Enzyme inhibitors are used to gain information about the shape of active site of enzyme and amino acids residues in active site.
- They are used to gain information about regulation or control of metabolic pathway.
- They can be used for drug designing.
- They are important for correcting metabolic imbalance.
- They are used for designing herbicides, pesticides and for killing pathogen.
Types of enzyme inhibitors:
I. On the basis of specificity:
- Co-enzyme inhibitor:
- Inhibits co-enzymes only. E.g. cyanide hydrazine, hydroxyl amine inhibits co-enzyme pyridoxal phosphate.
- Ion-cofactor inhibitor:
- E.g. fluoride chelate Mg2+ ion of enolase enzyme.
- Prosthetic group inhibitor:
- E.g. cyanide inhibit Heme of cytochrome oxidase.
- Apoenzyme inhibitor:
- E.g. antibiotics
- Physiological modulator:
II. On the basis of origin:
- Natural enzyme inhibitor:
- E.g. Alfatoxin,
– amanitin
- E.g. Alfatoxin,
- Artificial enzyme inhibitor (synthetic):
- E.g. drugs
III. On the basis of whether the inhibition is reversible or irreversible
1. Reversible inhibition:
- The enzyme inhibition in which the enzymatic activity can be regained after removal of inhibitors.
- Types of reversible inhibition:
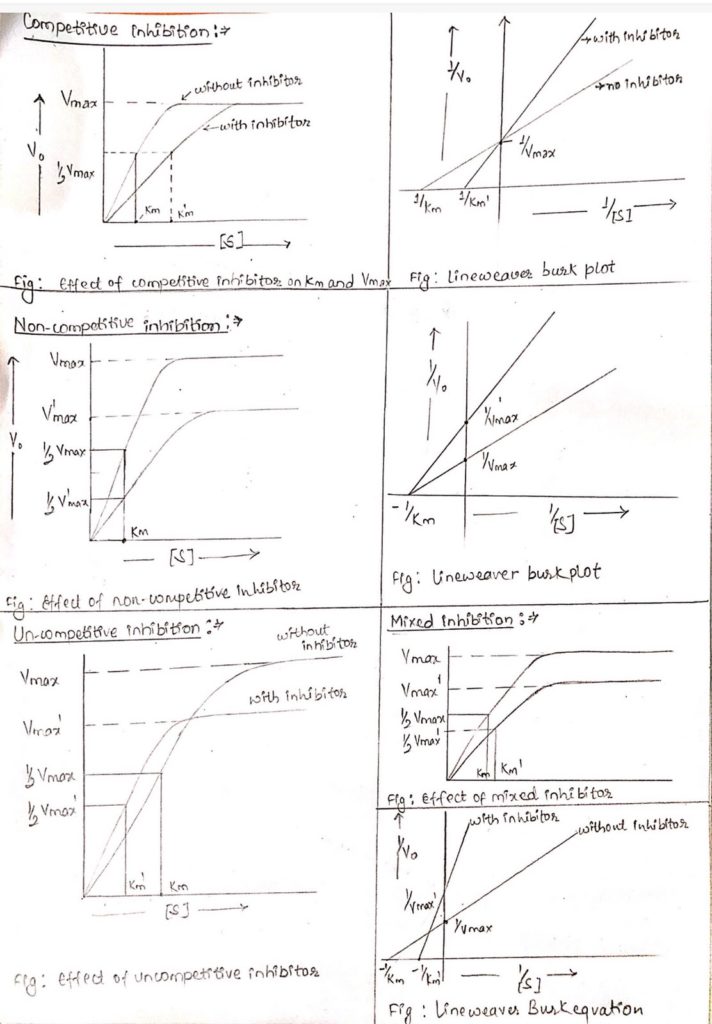
- i). Competitive inhibition
- Competitive inhibitors are substrate analog that bind to substrate binding site of enzyme i.e. active site so competition occurs between inhibitor and substrate for binding to enzyme.
- This type of inhibitor is overcome by increasing the concentration of substrate.
- The kinetics of reaction is Vmax remains same and Km increases.
- In this reaction, initially inhibitor binds to enzyme but with increase in concentration of substrate causes release of inhibitor.
- Then, substrate bind enzymes so that the Vmax remains same while Km increases.
- Example:
- Succinate dehydrogenase convert succinate to fumarate.
Succinate —succinate dehydrogenase————–> Fumarate + NADH +H+ - Malate is competitive inhibitor of succinate due to structural analogy.
- Malate + NAD+ —–succinate dehydrogenase———> Oxaloacetate
- Sulphonamide is competitive inhibitor of PABA during tetrahydrofolate synthesis.
- Succinate dehydrogenase convert succinate to fumarate.
- Example:
- Treatment of methanol poisoning:
- Methanol —–alcohol dehydrogenase————-> Formaldehyde (toxic)
- Ethanol ——alcohol dehydrogenase———> Acetaldehyde
- ii). Non-competitive inhibition:
- In this inhibition, there is no competition between substrate and inhibitor because the inhibitor binds to enzyme other than substrate binding site.
- Since the binding site of substrate and inhibitor to enzyme is different, inhibitor don’t affect the affinity of enzyme to substrate.
- In this case, the inhibition cannot be overcome by increasing substrate concentration.
- The kinetic reaction is Vmax decreases and Km remains same. This means that substrate concentration has no effect on inhibition.
- Binding of substrate and inhibitor are equal.
- The inhibitor changes the conformation of enzyme after binding so that substrate cannot bind to enzyme.
- This results in decrease of Vmax.
- Example:
- Heavy metal poisoning. Hg, Pb etc. distort the -SH group containing enzyme at allosteric site.
- Deoxycycline is non-competitive inhibitor of proteinase enzyme of bacteria.
- The non-competitive inhibitor can be removed by pH treatment or by hydrolysis.
- In case of metal poisoning, chelator is used.
- iii). Uncompetitive inhibitor:
- This type of inhibition is seen in multi-substrate reaction.
- It is rare type of inhibition.
- The process of inhibition is same as non-competitive but it only binds to ES-complex.
- At first substrate binds to enzyme to form ES-complex.
- After binding of substrate to active site of enzyme, the binding site for inhibitor forms at allosteric site so that inhibitor bind.
- The binding of inhibitor distorts the active as well as allosteric site of enzyme, inhibiting catalysis.
- In this inhibition, Vmax as well as Km both decreases.
- Examples:
- Inhibition of lactate dehydrogenase by oxalate.
- Inhibition of alkaline phosphatase by L-phenylalanine.
- iv. Mixed inhibition:
- This type of inhibition is commonly seen in multi-substrate reaction.
- It is the combination of competitive as well as non-competitive inhibition.
- The mixed inhibitor can bind to both active site and allosteric site.
- The kinetics of reaction is Vmax decreases and Km increases.
- The Vmax decreases because inhibitor non-competitively bind to allosteric site and distort enzyme.
- Similarly, Km increases because inhibitor can also bind to active site competiting with substrate.
- This type of inhibition cannot be removed by increasing substrate concentration.
- Examples:
- Ketoconazole is mixed inhibitor bind to 5
–α reductase enzyme.
- Pallidium ion is mixed inhibitor of oxidoreductase enzyme.
- Ketoconazole is mixed inhibitor bind to 5
2. Irreversible inhibition:
- In this type of inhibition, inhibitor bind to functional group of active sites by strong bond such as covalent bond and permanently destroy the catalytic property of enzyme.
- The functional group of active sites are -OH, -SH, -NH2, etc.
- The irreversible inhibitor is non-specific and cause dead end of enzyme activity.
- The inhibitor can bind free enzyme and ES complex and destroy it permanently.
- Examples:
- Iodoacetamide (CH2ICOONH2) bind with -SH group of enzymes permanently.
- Enzyme-SH + CH2ICOONH2 —-> Enzyme-SCH2COONH2 + HI
