
Fehling’s Test: Objective, Principle, Reagents, Procedure and Result
Objective:
- to detect reducing sugar in a given solution
Principle of Fehling’s test:
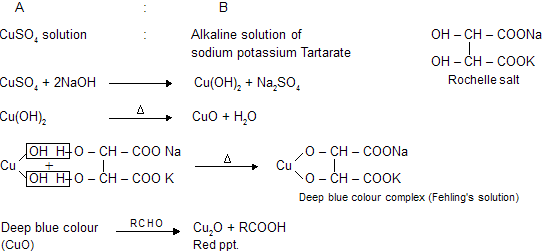
Fehling’s test is one of the sensitive test for detection of reducing sugars. Fehling’s reagents comprises of two solution Fehling’s solution A and solution B. Fehling’s solution A is aqueous copper sulphate and Fehling’s solution B is alkaline sodium potassium tartarate ( Rochelle salt). Rochelle salts (sodium potassium tartarate) present in the reagent acts as the chelating agent in this reaction.These two solution are mixed in equal amount before test.
On heating an aldehyde or reducing sugar with Fehling’s solution give reddish brown prepitate. Formation of red precipitate of cuprous oxide denotes the presence of reducing sugar.
Reagents:
- test solution: 5 % Glucose, 5 % Sucrose, 5 % fructose, 5 % Lactose, 5 % Starch
- Fehling’s reagent (solution A: CuSO4.5H2O
- Fehling’s reagent ( solution B: Sodium potassium tartrate)
- Water bath
- Pipettes
- Dry test tubes
Procedure of Fehling’s test:
- Take 1ml of sample in dry test tube.
- Take 1ml of distilled water in another tube as control.
- Add 1ml of Fehling’s reagent (A and B) to all the tubes.
- Keep in boiling water bath.
- Look for the development of red precipitate.
Result interpretation:
- Positive Fehling’s test: reddish brown ppt ( glucose, fructose, lactose)
- Negative Fehling’s test: No red ppt (sucrose, starch)

