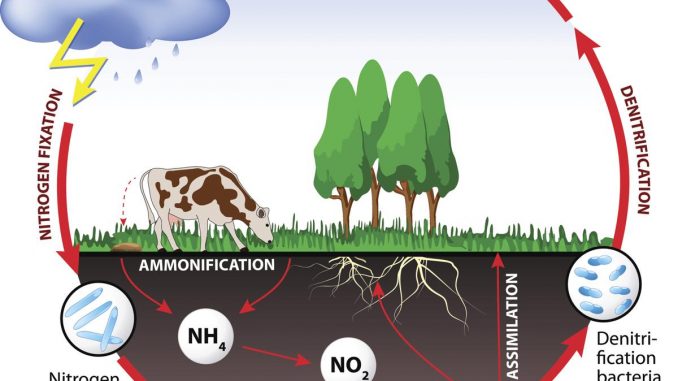
Nitrogen cycle: Steps of Nitrogen cycle
- Nitrogen undergoes a number of transformation that involves synthesis of organic compounds (amino acids, proteins, enzymes, chlorophylls, nucleic acids) as well as inorganic and volatile compounds (Ammonia, Nitrates, nitric acid). These transformation occurs simultaneously.
- N2 gas account for about 78% of air. There is very few uses of gaseous nitrogen. However, nitrogen is the most essential elements.
- Small parts atmospheric nitrogen is converted into organic nitrogen by certain free living and symbiotic nitrogen fixing microorganisms. Similarly, the nitrogen present in organic fraction of living beings is converted into ammonia (NH3) which is in turn utilized by many microorganisms or oxidized into nitrate. The nitrate may be lost into soil by leaching, may be used by plants or may be converted into atmospheric nitrogen.
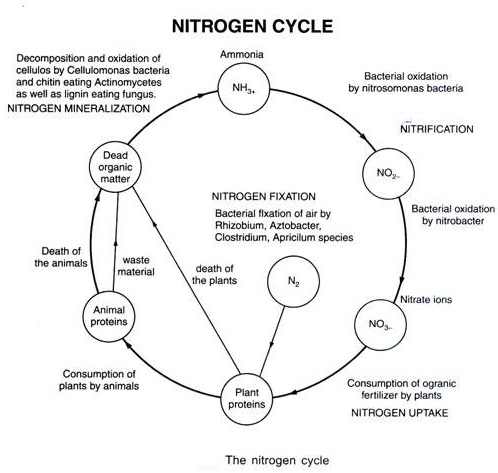
The whole transformation of Nitrogen into different form is known as Nitrogen cycle
It involves following process.
- Nitrogen fixation
- Nitrification
- Nitrogen assimilation/Mineralization
- Ammonification
- Denitrification
1. Nitrogen fixation:
- i. Biological nitrogen fixation
- ii. Non-biological nitrogen fixation
i. Biological Nitrogen Fixation
- Small part of atmospheric nitrogen (Nitrogen gas) is converted into biologically acceptable nitrogenous compound (NH3) by by biological organisms such as bacteria, BGA etc. and the process is called biological nitrogen fixation.
- Two groups of organisms are involved in this process- Symbiotic Nitrogen fixer and Non-Symbiotic Nitrogen fixer.
Symbiotic Nitrogen Fixation:
- Some bacteria living symbiotically in root nodules of legume plants can fix atmospheric nitrogen and make available for the plants. Eg. Rhizobium spp
- Some other symbiotic nitrogen fixers are:
- Rhizobium leguminosarum: pea
- Rhizobium phaseoli: Bean
- Rhizobium lupine: soyabean
- Bradyrhizobium japonicum: cow pea
Non-symbiotic Nitrogen Fixation:
- Several free living microorganisms fix atmospheric nitrogen into ammonia.
- Nitrogen fixing bacteria produces an enzyme complex known as nitrogenase that converts N2 into NH3.
- *Nitrogenase enzyme is composed of two sub-units ie. Dinitrogenase (MoFe containing protein) and dinitrogen reductase (Fe containing protein)
- The overall reaction of atmospheric nitrogen fixation by non symbiotic bacteria is absorption and reduction of atmospheric N2 into NH3.
- Many aerobic, anaerobic and facultative anaerobic bacteria as well as blue green algae can fix nitrogen non-symbiotically.
- Some Non-symbiotic (free living) nitrogen fixers:
- Aerobic bacteria: Azotobacter, Azomonas, Beijerinekia, Derxia etc
- Anaerobic bacteria: Clostridium, Desulfotomaculum, Desulfovibrio etc
- Photosynthetic bacteria: Rhodopseudomonas, Rhodomicrobium etc
- Blue green algae: Nostoc, Anaebaena, Calothrix, Oscilataria etc.
ii. Non-biological Nitrogen fixation:
- Atmospheric nitrogen react with Oxygen during lightening and thundering to produce nitrogen oxide. Then the nitrogen oxide get dissolved with rain water and fall to the ground, reacts with minerals in soil to form nitrates and ammonium salts.
- Haber’s Process: Nitrogen and hydrogen reacted at high temperature and pressure to form ammonia.
N2 + O2 —————–> NO2
NO2 + O —————-> N2O5
N2O5 + H2O—————-> 2HNO3
2HNO3 + CaCO3———-> Ca(NO3)2 + CO2 + H2O
2. Nitrification:
- Biological formation of nitrate and nitrite from ammonia is called Nitrification.
- Nitrate is produced not only in soil by nitrifying bacteria but also in marine environment and sewage treatment plant.
- The process of nitrification is especially important in soil because the transformation of NH4+ ion into NO3- ion results in the change in charge from positive (+ve) to negative (-ve). The positively charged ion tends to bound with negatively charged clay particles in soil while negatively charged NO3- ion tends to freely migrate in soil water so plants can radially take up nitrate ion by roots for assimilation into organic compounds.
- Some chemoautotropic bacteria are nitrifying bacteria which acquire energy from inorganic nitrogen compound.
- There are two groups of nitrifying bacteria:
- i. Ammonia oxidizer: these bacteria derive energy for cell synthesis by oxidation of ammonia and converts NH3 into nitrite. Examples Nitrosomonas, Nitrococcus, Nitrospira, Nitrosolobus
- ii. Nitrate oxidizer: these bacteria derive their energy by oxidation of nitrite. Example: Nitrobacter
3. Nitrogen assimilation and mineralization:
- Inorganic nitrogen compound present in soil after nitrogen fixation are absorbed by plants as nutrients and metabolize them for biosynthesis of aminoacids, enzymes, nucleic acids etc. the process is known as Nitrogen assimilation.
- When animal feeds on plants, nitrogen is deposited in the form of protein as well as converted in to other form such as urea, uric acids and excreted as faeces and urine.
- Similarly, some of the nitrogen compound undergoes mineralization and get deposited in the soil as ammonium salts.
4. Ammonification/ Decomposition of nitrogenous compound:
- Dead remains of plants and animals are decomposed by microorganisms (bacteria and Fungi) present in soil and convert the organic nitrogen compound into ammonia.
- Some Bacteria (Pseudomonas, Bacillus, clostridium, Serratia), fungi (Alternaria, Aspergillus, Mucor, Penicillium) and Actinomycetes (Streptomyces) can convert organic nitrogen compound into ammonia.
5. De-nitrification:
- The biological conversion of nitrite and nitrate into nitrous oxide or molecular nitrogen gas is known as de-nitrification. It is also known as Nitrate respiration.
- at first nitrate is reduced to nitrite which is then reduced to nitric oxide, then nitric oxide is reduced to nitrous oxide and finally to molecular nitrogen.
- De-nitrification cause loss of soil nitrogen into atmosphere. This process is known as volatilization of nitrogen.
- Denitrifying bacteria are anaerobes.
- Some examples of Denitrifying bacteria are: Pseudomonas denitrificans, Bacillus licheniformis, Thiobacillus denitrificans, Hypomicrobium, Chromobacterium etc
