
What is primary cell culture?
- The stage of the culture after isolation of the cells but prior to the first sub culture after which it becomes a cell line is termed as primary culture.
- It refers to that stage of the culture after the isolation of cells from tissue, where the cells are proliferated under appropriate conditions until they occupy all of the available substrate.
- There are 4 main stages:
- Acquisition of the sample
- Isolation of the tissue/explant
- Dissection and disaggregation
- Culture after seeding into the culture vessel
Isolation of tissue/explant:
- During the explant collection from animal or human sources, ethical issues/animal rights should be taken care of.
- The human explants collection should be done only after the consent from donor and their relatives.
- Work with human tissue should be performed at Containment Level 2 in a class II biological safety cabinet.
- Use 70% alcohol to sterilize the site of the resection if it is likely to be contaminated.
- Aseptically remove the tissue and transfer it to the tissue culture laboratory in dissection BSS (DBSS) or collection medium.
- To avoid contamination, follow protocols strictly.
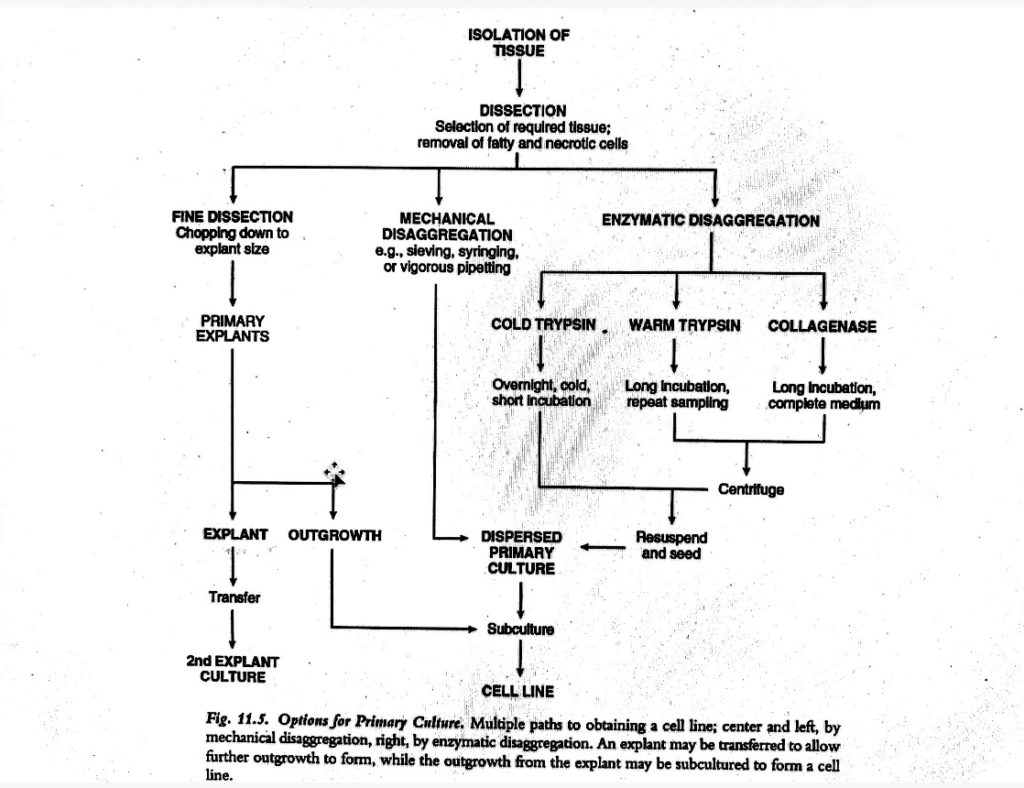
Disaggregation of tissue:
i. Mechanical disaggregation:
- The outgrowth of cells from primary explant is a relatively slow-going process and can be highly selective.
- Even if the enzymatic digestion has potential to give a culture which is more representative of the cells in the tissue, it is rather more strenuous.
- And due to the risk of proteolytic damage to cells during enzymatic digestion, mechanical disaggregation is chosen over it.
- Mechanical disaggregation includes spillage, sieving, syringing.
- Spillage: When the tissue is carefully sliced, the cells that spill out and the slices scraped are collected. This is known as spillage.
- Sieving: In this, the dissected tissue is pressed through a series of sieves which leads to reduced size of mesh.
- Syringing: In it, the tissue fragments is forced through a syringe (with or without a wide gauge needle), or simply pipetted repeatedly.
- This procedure yields a cell suspension more rapidly than the enzymatic digestion but may cause mechanical damage.
ii. Enzymatic disaggregation of tissue:
- A variety of glycopeptides like fibronectin and laminin mediates cell-cell adhesion in tissues (intercellular matrix and basement membranes).
- These glycopeptides are protease sensitive, thus enzymes can be used to disaggregate cells from tissues.
- Crude preparations of trypsin, elastase, pronase, collagenase, dispase, DNAse, hyaluronidase and heparinase, alone or in various combinations are most commonly used enzymes for tissue disaggregation.
- The simplest approach is to progress from a simple disaggregation solution to a more complex solution with trypsin alone or trypsin/ EDTA as an initiation point.
- Other proteases can be added or substituted to enhance disaggregation.
- Trypsin might be deleted, if necessary to increase viability.
- This technique results in higher number of the cells that are more representative of the whole tissue in less time.
- Protease and mechanical stress resistant cells are selected in dissociation techniques.
- In comparison to the newborn or adult tissues, the embryonic tissue disperses more readily and yields higher no. of proliferating cells.
- Crude trypsin is most frequently used enzyme as it is tolerated well by many cells and seems effective for many tissues.
- Warm Trypsinization method:
- In this technique, the minimization of exposure of cells to active trypsin is done in order to preserve maximum viability, thus dissociated cells are collected every half an hour.
- Trypsin is eliminated by centrifugation and is neutralized with serum in medium.
- It is useful for disaggregation of large amount of tissue in a comparatively short time, especially for chopped whole mouse embryos or chick embryos.
- It is not applicable for adult tissue containing lots of fibrous connective tissues.
- Sensitive cell types such as epithelium can be damaged by mechanical agitation.
- Cold trypsinization method:
- It is an easy method which minimizes the damage to cells during the exposure to trypsin.
- At 40 C, tissue is soaked in trypsin for 6-18hrs to allow penetration of the enzyme with little tryptic activity.
- It is followed by disaggregation at 37oC for 20-30 minutes.
- This technique provides a higher yield of viable cells, with enhanced survival after 24-h culture.
- It also preserves more variety of cell types than the warm method.
- As no stirring or centrifugation is involved, it is more convenient.
- Also, incubation at 40C may be done overnight.
- It is employed usually in case of soft tissues like embryonic cells.
- EDTA treatment:
- Cell adhesion molecules (CAMs) mediates in cell to cell adhesion.
- Some of these adhesion molecules are calcium dependent and thus sensitive to chelating agents like EDTA.
- Calcium is needed for tissues like epithelium for their integrity and thus can be easily disaggregated by treatment with EDTA prepared in balanced salt solution.
How to prepare primary chick embryo fibroblast (CEF) culture?
- Primary cultures of chick embryo fibroblasts (CEF) are extensively used for the cultivation of viruses.
- Some viruses may not grow in continuous cell lines, because of which the primary culture is still used.
Materials required:
- Fertile chicken eggs, 10 days old
- 70% (v/v) ethanol 1 X phosphate-buffered saline (PBS) with calcium and magnesium
- Versene solution
- 0.25% trypsin
- 1X complete EMEM
- 1X PBS-D
- Strong light source (75-100W)
- Small sterile beaker
- Sharp scissors and forceps
- Sterile petri dishes
- 50-ml conical tubes 37o C water bath
- 5-10 ml sterile pipettes
- 25 cm2 or 75 cm2 tissue culture flasks
Procedure of primary chick embryo fibroblast (CEF) culture
i. Remove embryo from egg
- Candle a 10-day old egg, by holding the egg in front of a bright light.
- Mark the position of the air sac with a pencil.
- Discard eggs with dead embryos or eggs without embryos.
- Use 70% ethanol to wash egg and place pointed-end-down in a small sterile beaker.
- Cut a large circular hole at the blunt end of the egg with a pair of sharp sterile scissors.
- Use sterile scissors or forceps to remove the shell above the air sac.
- Use sterile forceps to remove the membrane exposing the embryo.
- Use fresh sterile forceps to avoid cross-contamination.
- Gently and slowly remove the embryo so that it will free itself from the yolk and place it in a sterile petri dish.
- Care should be taken to not to touch the egg shell while removing the embryo.
ii. Prepare single-cell suspension from embryo:
- Use a fresh pair of sterile scissors to dissect the head, wings, feet, and body cavity contents from the embryo, and place into a 50ml conical tube.
- Remove contaminating blood/yolk by the addition and removal of 10ml of 1X PBS with calcium and magnesium.
- Macerate the embryo with dissecting scissors.
- Add 50ml of 1x PBS with calcium and magnesium to the tube and invert to wash the embryo. Allow the large pieces to settle to the bottom of the tube.
- Mix 25 ml of thawed 0.25% trypsin and 75 ml of versine solution and prewarm to 37oC.
- Remove buffer from the macerated embryo using a pipette and add 20 ml of this prewarmed solution.
- Shake the tube gently for 15-20 min at 37oC.
- Allow the large pieces to settle to the bottom of the tube.
- Use a pipette to transfer the supernatant, containing the cell suspension, to a tube containing 25ml of 1X complete EMEM. (Serum in the media will inactivate the trypsin).
- Centrifuge 10 min at 500 X g, room temperature.
- Remove the supernatant and resuspend the cell pellet in 20ml of 1 X complete EMEM. (The pellet will be loose, so care must be taken care when removing the supernatant.)
- Remove an aliquot of cell suspension and dilute 1:5 or 1:10 in PBS containing calcium and magnesium.
- Count cells using a hemacytometer.
iii. Culture of primary chick embryo fibroblast
- Prepare each 75-cm2 flask required by adding 1X 106 cells and 30 ml of 1 X complete EMEM. Begin incubation.
- Wash a confluent CEF monolayer once with 15ml of 1 X PBS-D per 75-cm2 flask.
- Mix 25ml of thawed 0.25% trypsin and 75 ml of versine solution and prewarm to 37oC.
- Decant PBS-D and add 5 ml of the trypsin solution to the monolayer per 75 cm2 flask.
- Incubate 5- to 10 min at room temperature until the cells begin to detach from the flask.
- Resuspend cells in the trypsin solution and disrupt cell clumps by pipetting up and down using a 5- or 10- ml pipette.
- Pipette cell solution into a conical tube of the appropriate size.
- Centrifuge 5 min at 500 X g, room temperature, to form a firm pellet.
- Decant supernatant and replace with the appropriate amount of 1 X complete EMEM medium. Resuspend cells by pipetting up and down.
- For preparation of a single 25 cm2 flask:
- Pipette a 1 ml aliquot of the cell suspension into 10 ml of 1 ml of 1X complete EMEM into a new 25 cm2 flask.
- For preparation of a large number of 25 cm2 flasks:
- In a sterile bottle, pipet 10 ml of 1X complete EMEM for each flask to be prepared.
- Add 1 ml cell suspension to this medium per each flask to be prepared.
- Pipette 10 ml cell/medium mixture into each new 25 cm2 flasks, swirling continuously.
- For preparation of a single 75 cm2 flask:
- Resuspend cells from a single 25 cm2 flask in 1 ml medium.
- Transfer to a 75 cm2 flask containing 30 ml of 1X complete EMEM.
- Alternatively, subculture a 75 cm2 flask into three 75 cm2 flasks or nine 25 cm2 flasks.
- Be careful while placing flasks in incubator with loosened caps and incubate 24 hr or until confluent.
- Freeze cells in cryotubes at ~106 cells/tube in 1 ml of freezing medium or subculture for experimental use.
