- Study about properties of protein within single cell is known as Proteomics.
Physical properties of amino acids
- Amino acids are colorless, crystalline substance.
- Most amino acids are tasteless but some are sweet. (E.g. Glycine, Alanine) and some are bitter (Eg. Arginine)
- Amino acids have high melting point (200-300)oC due to ionic property.
- Solubility:
- Solubility of amino acids depends upon polarity, iso-electric point, nature of solvent (pH) and temperature.
- Amino acids are soluble in water and ethanol (i.e. polar solvent) and insoluble in non-polar solvent like benzene, ether etc.
- Amino acids are insoluble at iso-electric point.
- Solubility depends upon pH of solvent and temperature.
- Eg. Tyrosine is soluble in hot water.
- Amphoteric property:
- Amino acids can act as acid and base due to their dipolar i.e. zwitter ion nature.
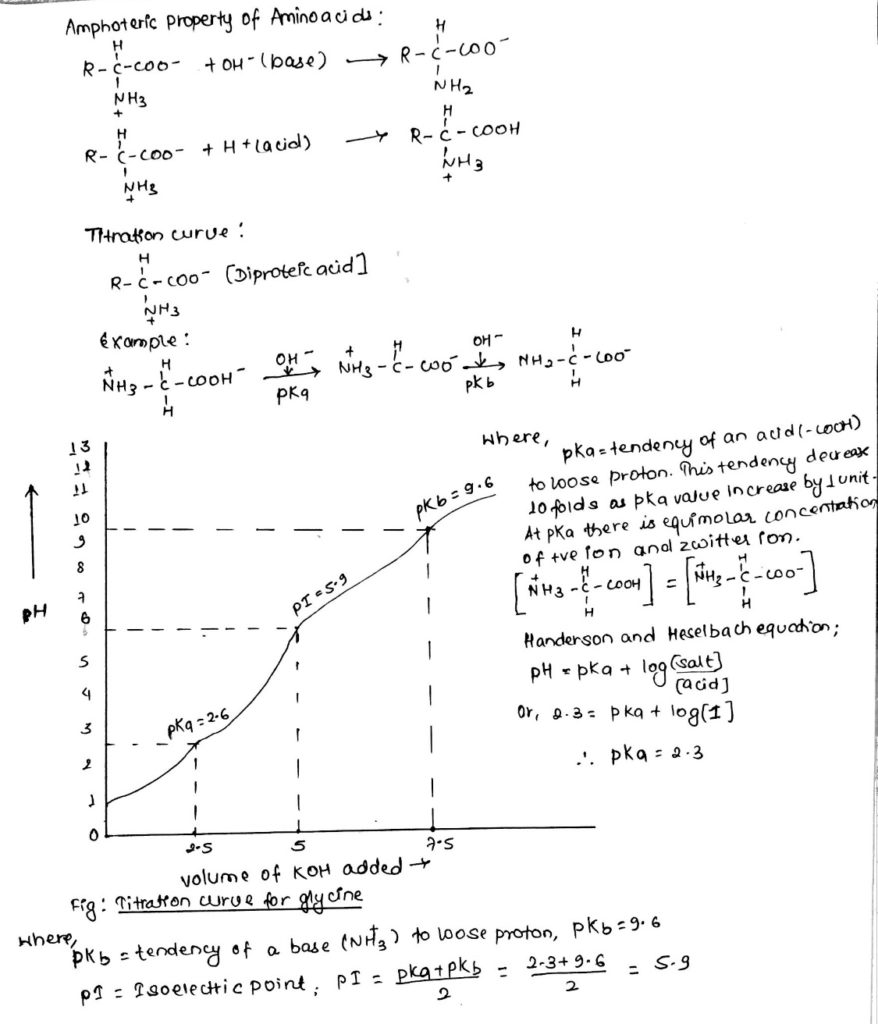
- Titration curve of amino acids:
- Most of the amino acids are monoamino-monocarboxylic acid.
- When they are in fully protonated form they can be titrated twice.
- Titration curve is the graph made between pH of amino acids and volume of acid or base added. It is always sigmoidal.
- pKa= tendency of an acid (-COOH) to loose proton.
- This tendency decreases 10 folds as pKa value increase by 1 unit.
- At pKa, there is equimolar concentration of +ve ion and zwitter ion.
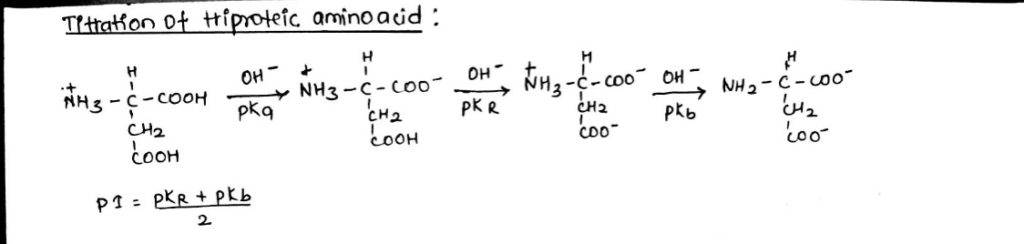
Important information from titration curve:
- pKa, pKb and pI value can be calculated.
- All the monoamino-monocarboxylic aminoacids (diproteic) have nearly about pKa and pkb values.
- Buffering zone (pKa+/-1) or (pKb+/-1).
- Glycine: pKa=2.3, pKb=9.6
- Alanine: pKa= 2.26, pKb=9.4
- Aspartic acid: pKa+2.09, pKb=9.82, pKR=3.86
- Histidine: pKa= 1.82, pKb=8.95, pKR=10.53
- Note:
- The α-carbonyl group of mono-amino-mono carboxylic amino acids is stronger acid than carboxyl group of the aliphatic acid.
- It is because of amino group and its +ve charge which increase tendency of carboxyl group to dissociate the -ve charge.
- Similarly, the α amino group of diproteic amino acid is stronger base than amino group of comparable aliphatic amines. It is because of presence of carboxyl group.
- Absorption spectrum:
- Amino acids absorbs the light at 280nm so the concentration of amino acids can be measured.
- Isomerism in amino acids:
- Except glycine, all amino acids exists in super impossible mirror image i.e. D and L form.
- This configuration is given by Emil Fischer.
- This configuration indicate absolute concentration but not indicate the optical activity of amino acids.
- – CHO group can be converted into -COOH group so they are compared.
- -OH group compared with -NH2 group.
- -R- group compared with -CH2OH group.
Chemical properties of amino acids:
- Why chemical properties of amino acid is importance?
- Chemical reactions of amino acids are important:
- For identification and analysis of amino acids in protein.
- For identification of amino acid sequences in protein.
- For identification of specific amino acid residue of native protein that are required for biological functioning e.g. haemoglobin (Histidine has role in it).
- For chemical modification of amino acids residue in protein molecules to produce change in biological activity.
- For chemical synthesis of the polypeptides (for medical purpose).
Types of chemical reactions given by amino acids:
- Reaction due to -COOH group.
- Reaction due to -NH2 group
- Reaction due to both
- Reaction due to R-group
1. Chemical reactions of amino acids due to -COOH group:

2. Chemical reactions of amino acids due to both -COOH and -NH2 group:
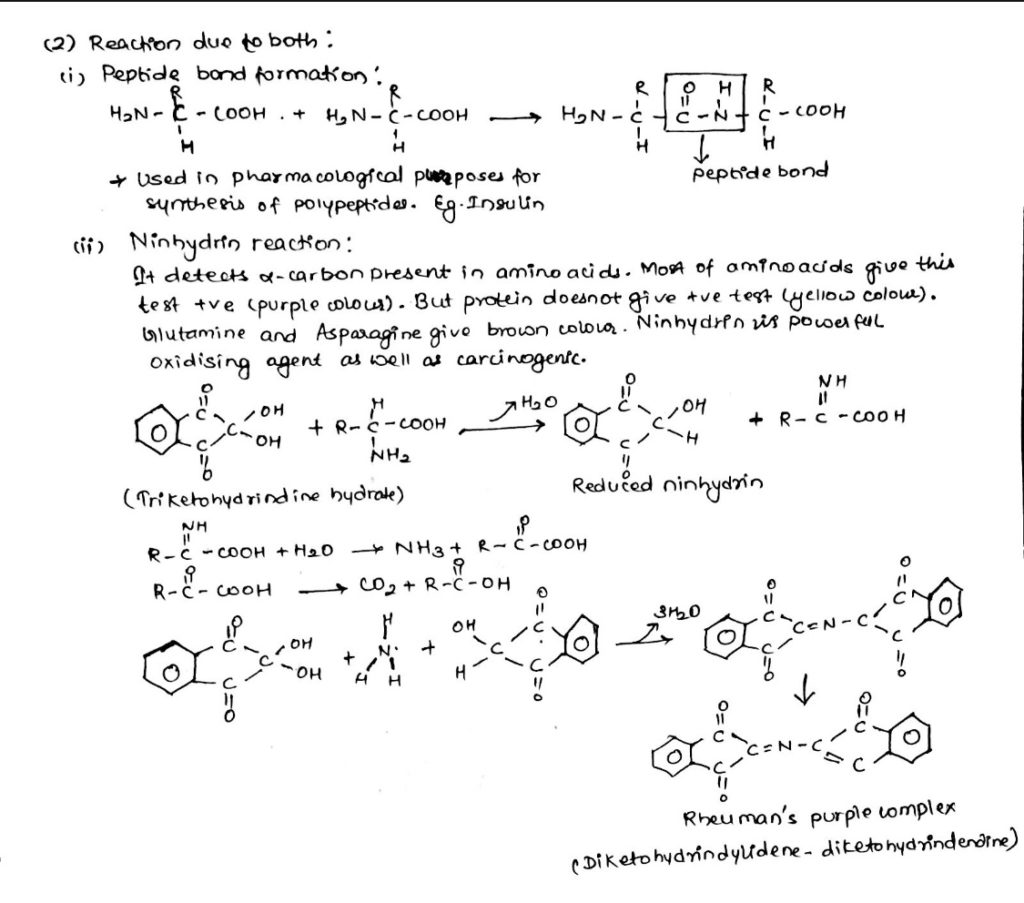
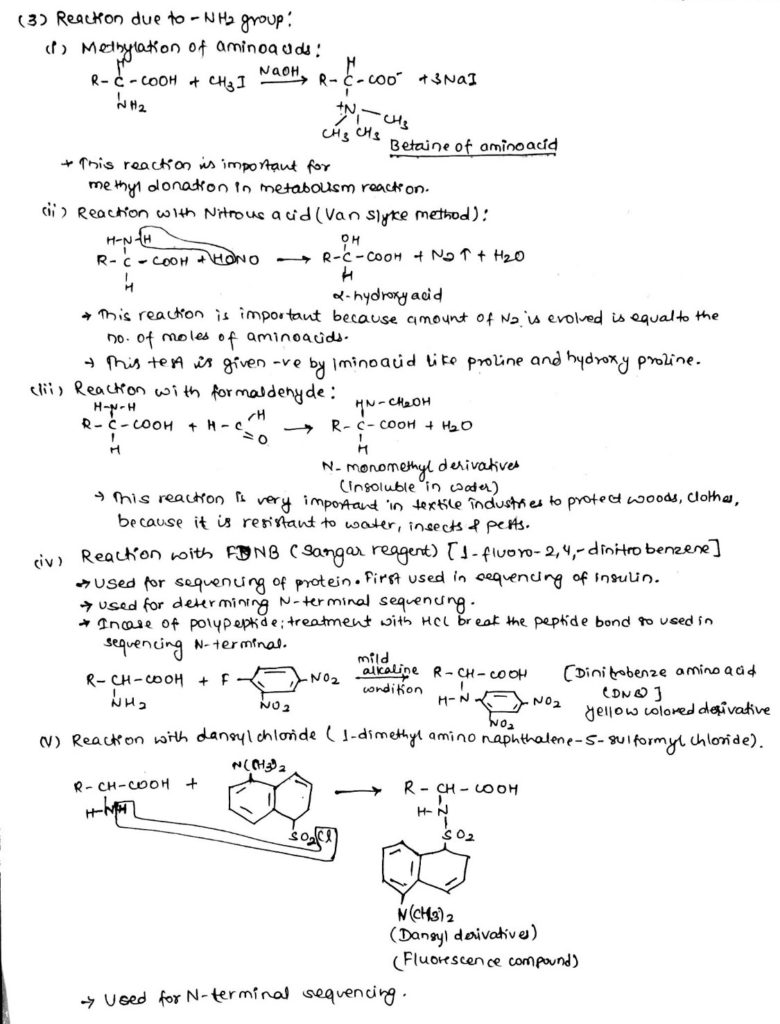
3. Chemical reaction of amino acids due to -NH2 group
4. Chemical reaction of amino acids due to functional group:
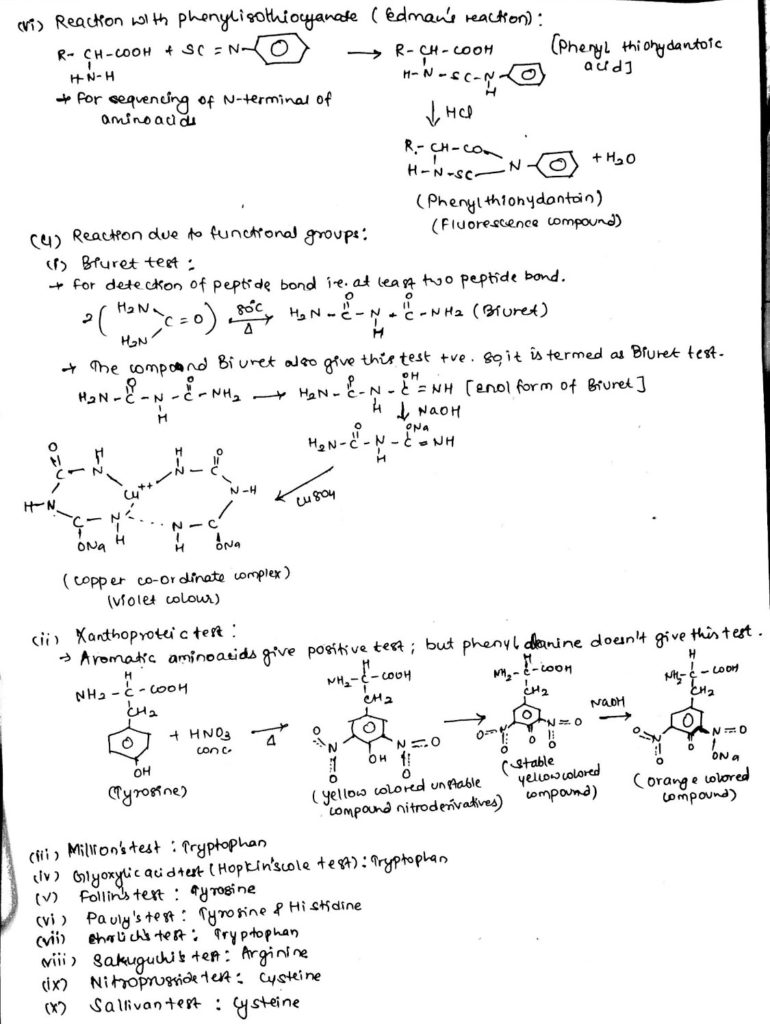
Functions of amino acids:
- Precursor for synthesis of proteins and polypeptides.
- Used for glucogenic and ketogenic degradation.
- For synthesis of porphyrin.
- For synthesis of melanin: Melanin is complex polymeric structure made up of tyrosine and also may contain tryptophan.
- For synthesis of creatine and creatine phosphate.
- For synthesis of plant hormones (auxin).
- For synthesis of neurotransmitter.
- For synthesis of animal hormones (thyroid- thyroxine)
- For synthesis of lignin, tannin, papaverine (alkaloids).
- For synthesis of vitamins (Ascorbic acid), niacin.
- Synthesis of antibacterial agents (penicillin G).
