T-cell maturation:
- The migration of progenitor T-cells from the early sites of hematopoiesis to the thymus takes place at about day 11 of gestation in mice and in 8th or 9th week of gestation in humans.
- T-cell maturation involves the re-arrangement of the germ-line TCR genes and the expression of various membrane markers.
- In thymus, the developing T cells are termed as thymocytes.
- These thymocytes proliferates and differentiates along developmental pathways that produce functionally distinct sub-population of mature T-cells.
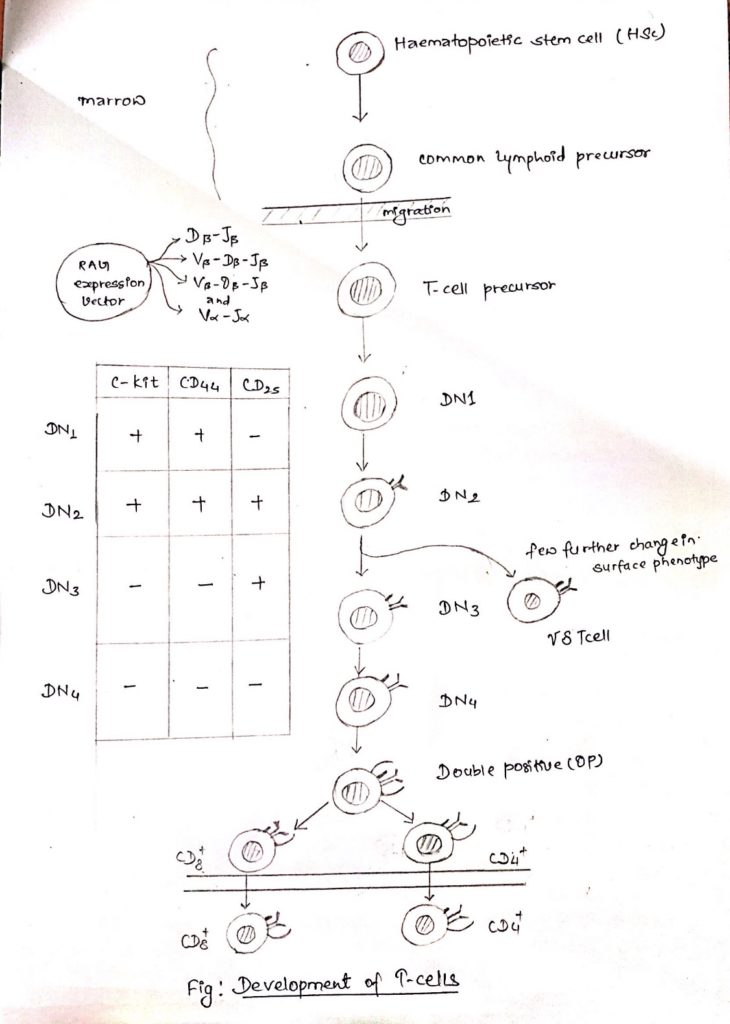
- T-cells development initiates with the arrival of small numbers of lymphoid precursors migrating from the blood into the thymus where they proliferate, differentiate and undergo selection process that result in the development of mature T cells.
- When T-cells precursor arrive at thymus, they don’t express the signature surface markers of T cell as the T-cell receptors, the CD3 complex or the co-receptors CD4 and CD8.
- In-fact these progenitor cells have not yet re-arranged their TCR genes and do not express the proteins, such as RAG-1 and RAG-2 that are required for re-arrangement.
- After arriving in the thymus T cell precursors enter the cortex and slowly proliferate.
- The differentiating T-cell pass through a series of stages that are marked by characteristic changes in their cell surface phenotype, during approximately three weeks of development in thymus.
- The thymocytes early in the development lack detectable CD4 AND CD8.
- As these cells are CD4-, CD8-, they are termed as double negative (DN) cells.
- In-fact DN-T cells can be sub-divided into 4 subsets (DN1-4) characterized by the presence or absence of cell surface molecules in addition to CD4 and CD8, such as:
- C-kit, the receptor for stem cell growth factors
- CD44, an adhesion molecule
- CD25, it is the alpha chain of the IL-2 receptor
- The cells that enter the thymus DN1 cells are capable of giving rise to all the subsets of T cells and are phenotypically C-kit+, CD44 high and CD25-.
- Once the DN1 cells encounter the thymic environment, they begin to proliferate and express CD25 becoming C-kit+, CD44 high and CD25+.
- These cells are called DN2 cells.
- During the critical DN2 stage of development, rearrangement of the genes for the TCR, Ƴ, 𝛿 and β chain begins.
- However, the TCR α locus does not re-arrange, presumably because the region of DNA encoding TCR α gene is densely compacted and not accessible to recombinase machinery.
- As cells progress to DN3, the expression of both C-kit and CD44 is turned off and TCR Ƴ, TCR 𝛿 , and TCR β re-arrangement progresses.
- Cells destined to become Ƴ𝛿 T cell, diverge at the transition between DN2 and DN3 and become mature.
- Ƴ𝛿 T cell which vary few changes in their surface phenotype most DN2 cells are destined to give rise to T cells.
- On assuming the DN3 phenotype (C-kit–, CD44– and CD25+), the cells halt proliferation and protein products of TCR β rearrangements are detected in the cytoplasm of these cells.
- The newly synthesized β-chain combine with 33-kDa glycoprotein known as the pre-T cell receptor or pre-TCR.
- Formation of pre-TCR activates a signal transduction pathway that has the following consequences:
- It indicates that a cell has made a productive TCR β-chain re-arrangement and signals its further proliferation and maturation.
- It suppresses further re-arrangement of TCR β-chain gives resulting in the allelic exclusion.
- It renders the cell permissive for re-arrangement of the TCR α chain.
- It induces developmental progression to CD4+ and CD8+ double positive (DP) state.
- After β-chain rearrangement is completed the DN3 cells quickly progress to DN4, the level of CD25 falls and both CD4 and CD8 co-receptors are expressed.
- Thus, double positive (DP) stage is a period of rapid proliferation.
- However, TCR α-chain gene rearrangement still has not occurred at this stage of time.
- The rearrangement of α-chain gene does not begin until the double positive (DP) thymocytes stops proliferating and RAG-2 protein level increases.
- T-cell development is a costly process for the host.
- An estimated 98% of all thymocytes do not mature i.e. they die by apoptosis within the thymus either because they fail to make a productive TCR gene re-arrangement or because they fail to survive thymic selection.
- Double positive thymocytes that express αβ TCR CD3 complex and survive thymic selection develop into immature single positive CD4+ thymocyte or single positive CD8+ thymocytes.
- These single positive cells undergo additional negative selection and migrate from the cortex to medulla, where they pass from the thymus into the circulatory system.
- After the gene rearrangement, T-cell (Thymocytes) undergoes thymic selection.
Thymic selection of T-cell:
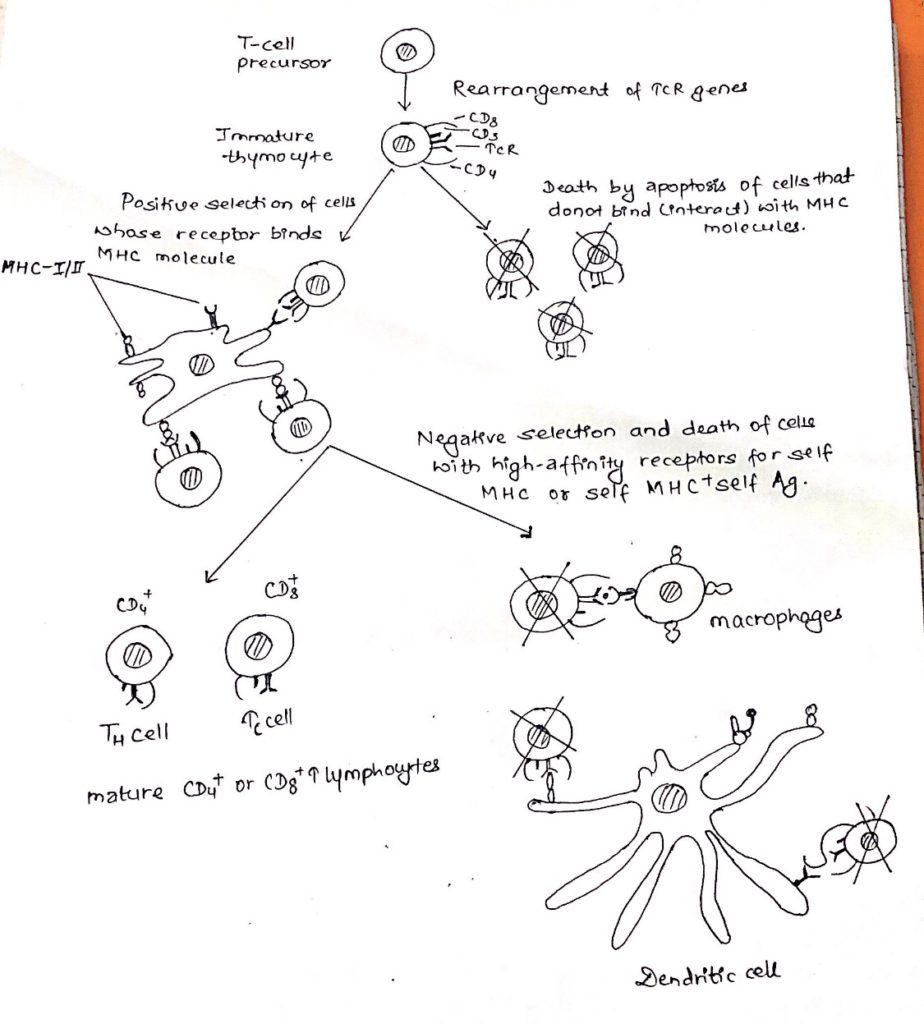
- The most characteristic property of mature T-cells is that they identify only foreign antigen combined with self MHC molecules.
- For this purpose, the thymocyte undergo two selection process in thymus.
- Positive selection:
- Positive selection occurs in the cortical region of the thymus.
- It involves the interaction of immature thymocytes with cortical epithelial cells.
- This interaction allows the immature thymocytes to receive a protective signal.
- This signal prevents them from undergoing cell death.
- Cells whose receptors are not able to bind MHC molecules would not encounter with the thymic epithelial cells and as a result it would not receive the protective signal resulting their death by apoptosis.
- This results in MHC restriction.
- Negative selection:
- The population of MHC restricted thymocyte that survive positive selection includes cells with receptors having a range of affinities from low to high for self-antigen presented by self-MHC molecule.
- Thymocytes with high affinity receptors are weeded out during negative selection via an interaction with thymic stomal cells.
- In case of negative selection, dendritic cells and macrophages having class I and class II MHC molecules interact with thymocyte bearing high affinity receptors for self MHC molecule alone.
- cells that undergo negative selection are observed to undergo death by apoptosis.
- Tolerance to self-antigens encountered in the thymus achieved by eliminating T-cells that are reactive to these antigens.
T-cell activation:
- The central event in the generation of both humoral and cell-mediated immune responses in the activation and clonal expansion of T-cells.
- T-cells activation is initiated by interaction of the TCR-CD3 complex with a processed antigenic peptide bound to either class-I (CD8+ cell) or class II (CD4+ cell) MHC molecules on the surface of an antigen presenting cells.
- The interaction and the resulting activating signals also involve various accessory membrane molecules on the T-cell and the antigen presenting cell.
- Interaction of T cell with antigen initiates cascade of biochemical event that induces the resting T-cell to enter the cell cycle, proliferating and differentiating into memory cells or effector cells.
- The key element in the initiation of T cell activation is the recognition by the TCR of MHC peptide complexes on antigen presenting cells.
- This event catalyses a series of intracellular events beginning at the inner surface of the plasma membrane and culminating in the nucleus, resulting in the transcription of genes that drive the cell cycle and/or differentiation of T-cell.
T-cell differentiation:
- CD4+ and CD8+ T-cells leave the thymus and enters the circulation as resting cells in the G0 stage of cell cycle.
- There are about twice as many CD4+ T-cells as CD8+ T-cells in the periphery.
- T cells that have not yet encountered antigen (naive T-cells) are characterized by condensed chromatin, very little cytoplasm, and little transcriptional activity.
- Naive T cells continually reticulate between the blood and the lymph system.
- During recirculation, naïve T cells reside in secondary lymphoid organs/tissues such as lymph nodes.
- If a naive cell does not encounter antigen in a lymph node, it exits through the efferent lymphatics ultimately draining into the thoracic duct and rejoining the blood.
- It is estimated that each naive T cell recirculates from blood to the lymph nodes and back again every 12-24 hrs.
- This large-scale recirculation increases the chances that naive T cell will encounter appropriate antigen because only about 1 in 105 naive T -cell is specific for any given antigen.
- If naive T cell recognize an antigen-MHC complex on an appropriate antigen cell or target cell, it will be activated, initiating a primary response.
- About 48hrs after activation the naïve T cells enlarges into a blast cell and begins undergoing repeated rounds of cell-division.
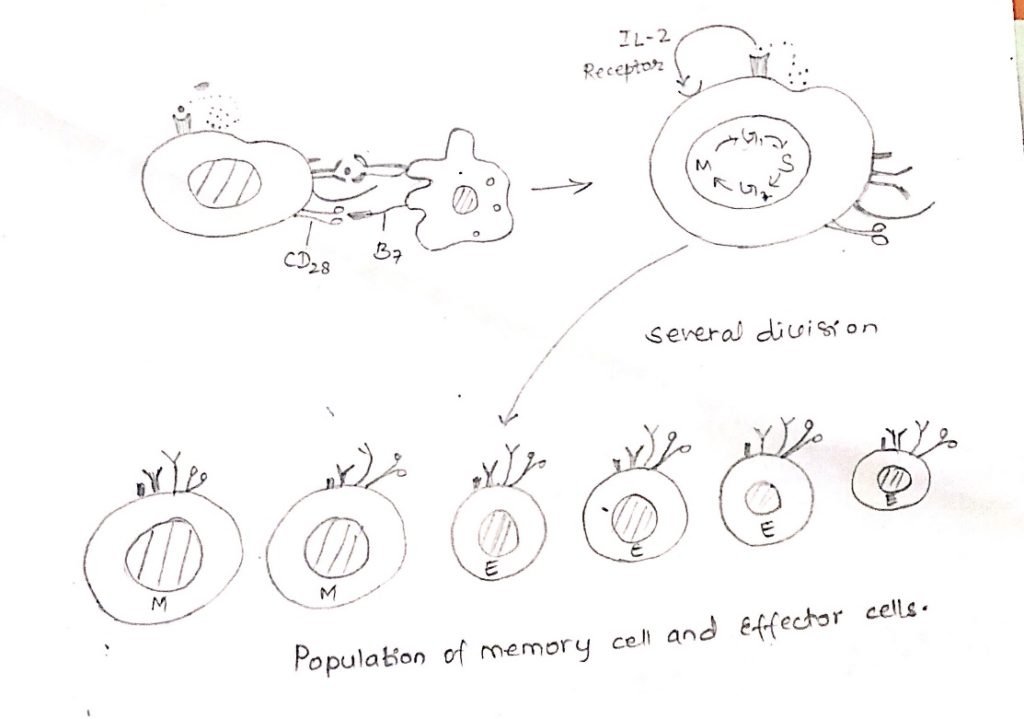
- Activation depends on a signal induced by the enlargement of the TCR complex and a stimulatory signal induced by the CD28-B7 interaction.
- These signals trigger entry of T cell into the G1 phase of the cell cycle and at the same time, induce transcription of the gene for IL-2 and α chain of the high affinity IL-2 receptor (CD25).
- Moreover, the co-stimulatory signal increases the half-life of the IL-2 mRNA.
- The increase in IL-2 transcription together with the stabilization of the IL-2 mRNA, increases IL-2 production by 100 folds in the activated T-cell.
- Secretion of IL-2 and its subsequent binding to the high affinity IL-2 receptor induces the activated naïve T cell to proliferate and differentiate.
- T-cells activated in this way divide 2-3 times per day for 4-5 days generating a clone of progeny cells which differentiates into memory or effectors T-cell populations.