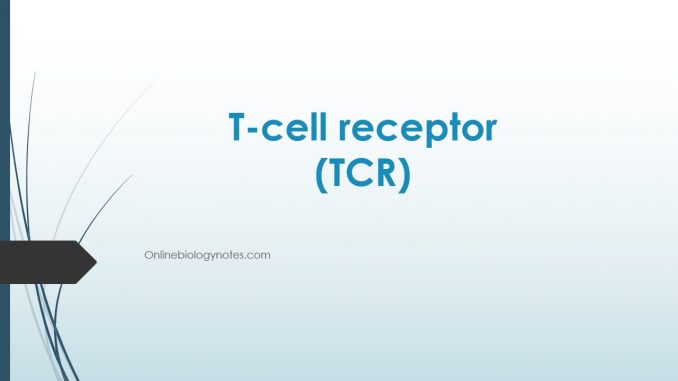
Structure of T-cell receptor (TCR):
- The domain structure of αβ and Ƴ𝛿 TCR heterodimers are stringly similar to those of immunologlobins; thus they are classified as members of immunoglobulin superfamily.
- Each chain of TCR has two domains containing an intra-chain disulfide bond that spans 60 to 75 amino acids.
- The amino terminal domain in both chains exhibits marked sequences variation but the sequences of the reminder of each chain are conserved. Thus the TCR domains- one variable (V) and one constant (C) are structurally homologous to the V and C domains of immunoglobulin and the TCR molecules resembles a Fab fragment attached to the cell membrane instead of to constant region of an Ig molecule.
- The TCR variable domains have three hyper-variable regions which appear to be equivalent to the complementarity determining regions (CDRs) in immunoglobulin light and heavy chains.
- Each TCR chain contains a short connecting sequence in which a cysteine residue forms a disulfide link with the other chain of the heterodimer.
- Following the connecting region is a trans-membrane region of 21 to 22 amino acids which anchors each chain in the plasma membrane.
- The trans-membrane domains of both chains are unusual in that they contains positively charged amino acids residues and these residues promotes interaction between the chain of the TCR heterodimer and chains of the signal-transducing CD3 complex.
- Each TCR chain contains a short cytoplasmic tail of 5-12 amino acids at the carboxyl terminal end.
- The majority of circulating T cells in human and mouse express T cell receptors (TCR) encoded by αβ genes.
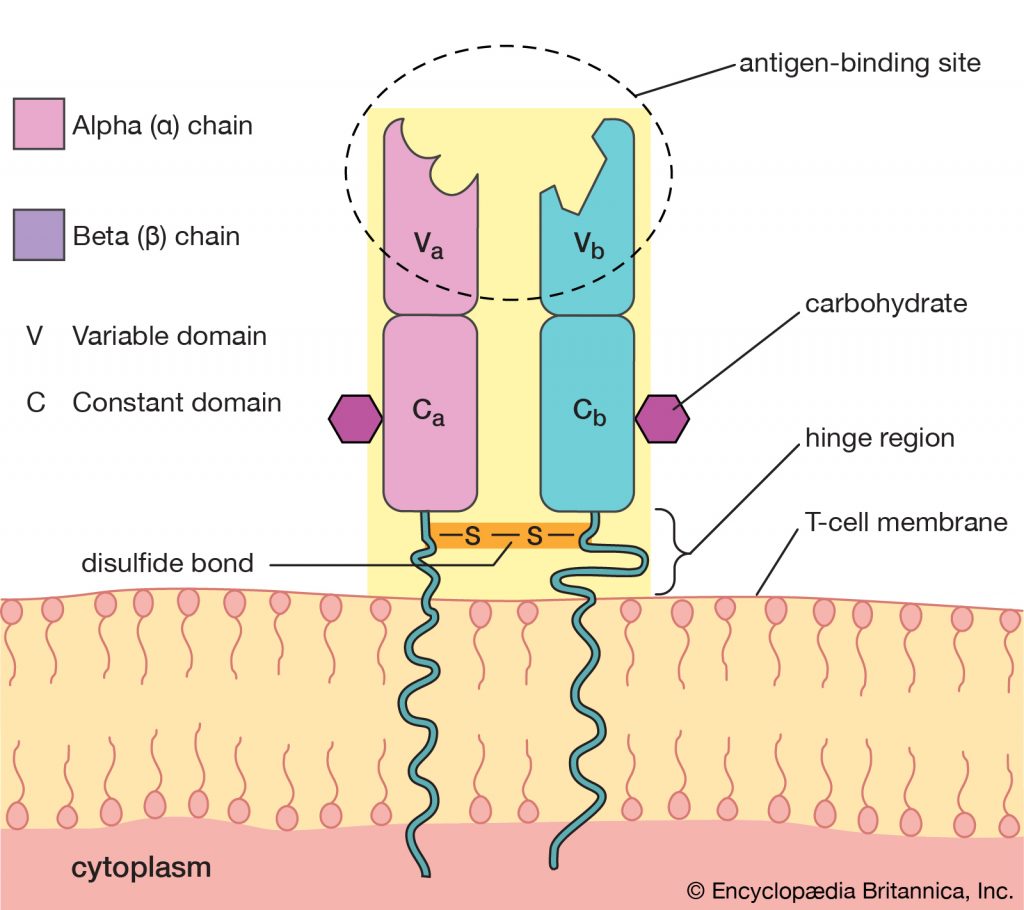
Role of TCR in antigen presentation:
- These TCR receptors encoded by αβ genes interact with peptide Ag processed and presented on antigen presenting cells (APCs).
- The remaining TCR were found to be Ƴ𝛿 which interacts with non-peptide antigen presented by the products of CD1 family of genes.
- Role of TCR in signal transduction however TCR itself cannot transduce the signal. For signal transduction TCR associates with CD3 molecule forming TCR-CD3 complex.
- The CD3 molecules participate in signal transduction after interaction of T-cell (TCR) with antigens. However CD3 does not influence interaction of antigen.
- CD3 is a complex of five invariant polypeptide chains that associate to form three dimers; a heterodimer of gamma and epsilon (Ƴε), a heterdomer of delta and epsilon chain (𝛿ε) and a homodimer of two zeta chains (ζζ) or heterodimer of zeta and eta chain (ζη).
- The ζ and η chains are encoded by same gene but differ in their carboxyl terminal ends because of difference in RNA splicing of the primary transcript.
- About 90% of the CD3 complexes examined to date incorporate the (ζζ) homodimer; the reminder have the (ζη) heterodimer.
TCR-CD3 complex:
- The TCR complex is pictured as four dimers; αβ or Ƴ𝛿 of TCR and Ƴε, 𝛿ε and ζζ or ζη of CD3
- The αβ or Ƴ𝛿 TCR heterodimer determines the ligand binding site with specificity
- The CD3 dimers ((Ƴε, 𝛿ε and ζζ or ζη) are required for membrane expression of the T cell receptor and for signal transduction.
- The Ƴ, 𝛿 and ε chains of CD3 are members of the immunoglobulin superfamily, each containing an immunoglobulin like extracellular domain followed by a trans-membrane region and a cytoplasmic domain of more than 40 amino acids.
- The zeta (ζ) chain has a distinctly different structure with a very short external region and a long cytoplasmic tail containing 113 amino acids.
- The trans-membrane region of all CD3 polypeptide chain contains a negatively charged amino acids residue (Aspartic acid and glutamic acid) that interacts with one or two positively charged amino acids residues in the trans-membrane region of each TCR chain.
- The cytoplasmic tails of the CD3 chain contains a motif called the immune-receptor tyrosine based activation motif (ITAM). The ITAM sites have been shown to interact with tyrosine kinases and to play an important role in signal transduction.
- In CD3 the Ƴ, 𝛿 and ε chain each contains a single copy of ITAM whereas the ζ and η chains contains three copies of ITAM.
Role of TCR-CD3 complex in antigen recognition:
- TCR-CD3 complex majorly recognizes the Ag-MHC molecules and various other membrane molecule play important role in Ag recognition and T-cell activation.
- Some of these membrane molecules strengthen interaction between T cell and APC or target cells and some act in signal transduction and some do both.
- CD4 and CD8 co-receptors binds to conserved region of MHC-II (α2, β2) or MHC-I (α3, β2 micro-globulin) molecules.
- CD4 is a 55KDa monomeric membrane glycoprotein that contains 4 extracellular immunoglobulin like domains (D1-D4), a hydrophobic trans-membrane region and a long cytoplasmic tail containing three serine residue that can be phosphorylated.
- CD8 generally takes the form of a disulfide linked αβ heterodimer or αα homodimer. Both α and β chain of CD8 are small glycoprotein of approximately 30 to 38 KDa. Each chain consists of a single extracellular immunoglobulin like domains a stalk region, a hydrophobic transmembrane are region and a cytoplasmic tail containing 25-27 residues several of which can be phosphorylated.
