Tests for specific carbohydrates: Seliwanoff’s test, Bial’s test and Iodine test
1. Seliwanoff’s test: Principle, reagents, procedure and result
Principle of Seliwanoff’s test:
Seliwanoff’s test is used to distinguish aldoses from ketoses. On treatment with conc. Acid, ketoses are dehydrated more rapidly to give furfural derivatives and on condensation with resorcinol give cherry red complex. The test will be answered by fructose, sucrose and other keto containing carbohydrates.
Reagents:
- test solution: 5 % Glucose, 5 % Sucrose, 5 % Fructose.
- Seliwanoff’s reagent (0.5% resorcinol in 3N HCl)
- Water bath
- Pipettes
- Dry test tubes
Procedures
- Take 1ml of sample in test tube and take 1ml of distilled water in another tube as control.
- Add 3ml of Seliwanoff’s reagents in both test tube
- Keep the test tubes in water bath for 1-2 minutes.
- Look for the development of red color.
**If the reaction is allowed for longer time, aldoses also produce positive results.
Result Interpretation of Seliwanoff’s test:
- Positive seliwanoff’s test: Fructose and sucrose
- Negative seliwanoff’s test: glucose, distilled water

2. Bial’s Test: Principle, reagents, Procedure and result
Principle of Bial’s test:
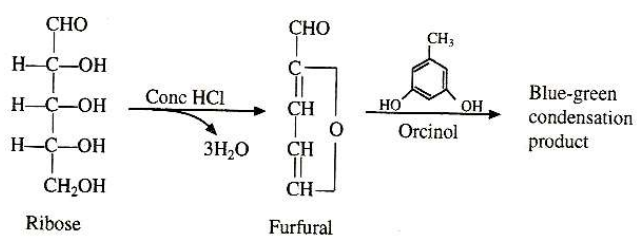
Bial’s test is useful in distinguishing pentoses sugar from hexoses sugars. Pentosses ( such as ribose sugar) form furfural in acidic medium which condense with orcinol in presence of ferric ion to give blue green colored complex which is soluble in butyl alcohol.
Reagents:
- test reagent: 5 % Glucose, 5 % Ribose, 5 % Fructose
- Bial’s reagent
- Water bath
- Dry test tubes
- Pipettes
Procedures of Bial’s test:
- Take 2ml of Bial’s reagent in test tube.
- Add 4-5 drops of test solution to this reagent.
- Keep in water bath for 30 seconds.
- Look for the development of bluish green color.
Result interpretation for Bial’s test:
- Positive Bial’s test: formation of blue color ( eg. Ribose sugar)
- Negative Bial’s test: formation of any other color indicates negative test. Hexose sugar ( glucose, fructose) generally gives green, red or brown color product.

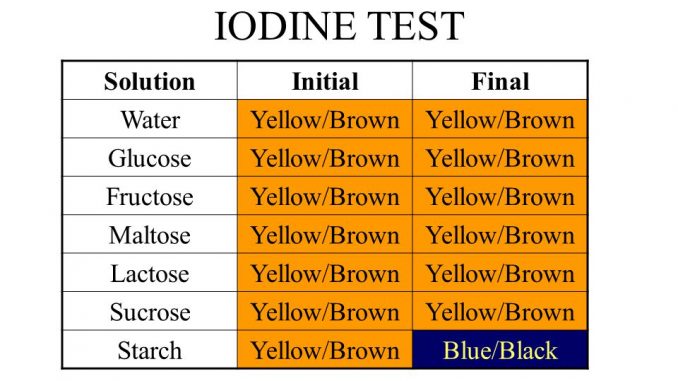
3. Iodine Test: Principle, reagents, Procedure and Result
Principle of Iodine test for carbohydrate:
Starch when reacted with I2 forms absorbed compound that gives blue color. On heating or on addition of alkali like NaOH or KIH, color disappears. This reaction is only physically association where I2 traps in the coiled structure of polysaccharide. On heating or on addition of alkali; the coiled structure becomes linear and the I2 molecules are released and the color disappears. The test will be answered by fructose, sucrose and other keto containing carbohydrates.
Reagents
- 5 % Glucose, 5 % Sucrose, 5 % Fructose, 5 % Starch
- Iodine solution
- Water bath
- Dry test tubes
- Pipettes
Procedure for Iodine test for carbohydrate
- Take 2ml of sample in test tube and take 2ml of distilled water in another tube as control.
- Add 5 drops of iodine solution to all the tubes.
- Look for the development of blue color.
- Heat the solution, the blue color will disappear and on cooling the color reappears.
Result interpretation of Iodine test:
- Positive iodine test: dark blue color (starch)
- Negative iodine test: glucose, fructose and sucrose